What is an SME or mid-cap?
The EU has a specific criteria for a SME or a mid-cap. Meeting this criteria is a prerequisite for getting the funding and it needs to be proven in cases when the funding is targeted only for SMEs or mid-caps.
Size is not everything
In determining whether or not an enterprise is an SME, the size of the enterprise (employees, turnover and balance sheet total) is not the only factor that should be taken into account. In fact, an enterprise can be very small in these terms, but if it has access to significant additional resources (e.g. because it is owned by, linked to or partnered with a larger enterprise), it might not be eligible for SME status.
For enterprises with a more complex structure, a case-by-case analysis may therefore be required to ensure that the enterprise matches with the requirements of the SME definition.
The definition of a mid-cap is somehow trickier as there is no official definition proposed by the European Commission. The EC broadly defines mid-caps as having fewer than 3,000 employees.
Since there is no official definition of mid-caps which would be used in all funding programmes and calls, the best advice is to stay up-to-date for EU funding programmes and check the eligibility criteria in each grant call.
Funding for R&D or market penetration?
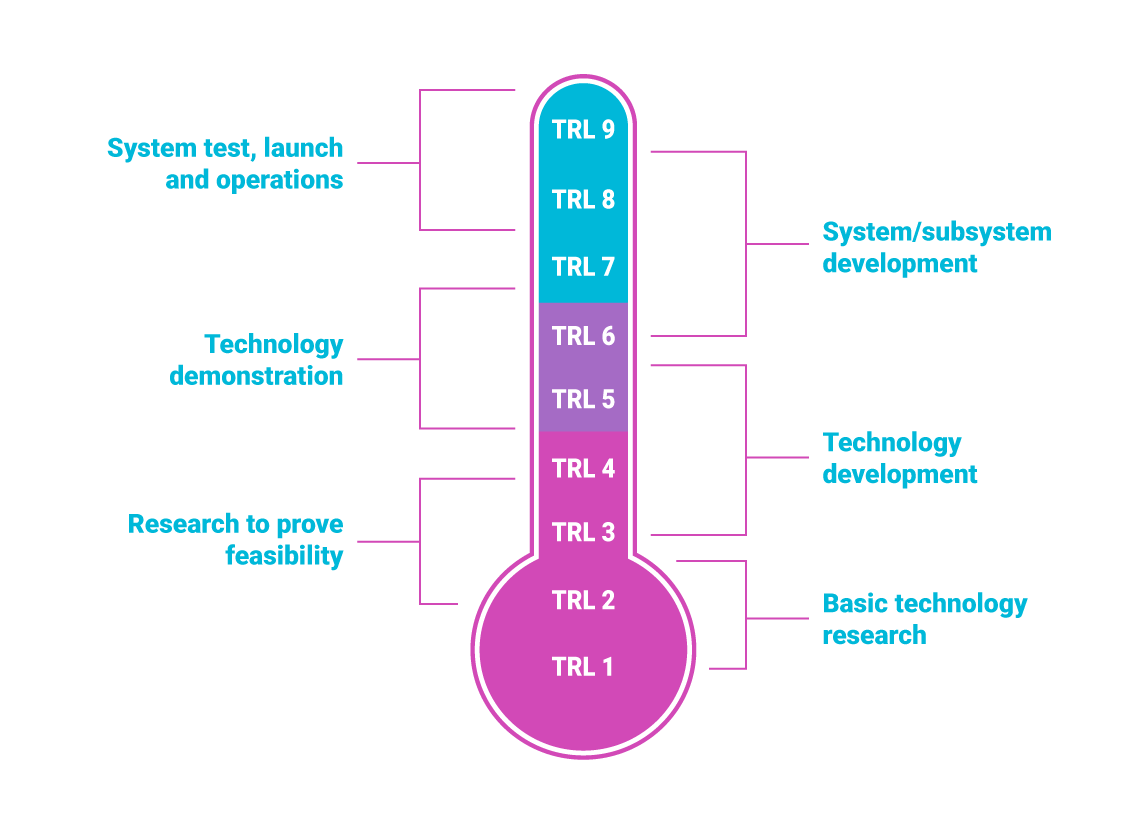
EU jargon often refers to something called TRL. This stands for Technology Readiness Level. TRLs are a method of estimating the maturity of technologies, originally developed by NASA in the 1970’s. The EU prefers to use the same technique in some of its funding instruments.
The projects that start from the lower levels of TRL (1-4) focus on research and development. The projects that are higher in the TRL scale, are usually for technology validation (TRL 4-6), close-to-market innovations and scaling up (TRL 6-9).
What KIND OF fUnding is available for each development stage?
How ready is your innovation?
Definition of this TRL level:
Software: To reach this TRL level, you need to have the basic principles of technology observed and reported, but applied research or development has not been initiated.
Medical research: The basic principles of technology are observed and reported, but applied research or development has not been initiated.
Examples of possible EU funding programmes: EIC Pathfinder of Horizon Europe
Definition of this TRL level:
Software: To reach this TRL level you have some basic principles observed and you have started R&D, but applications are still speculative.
Medical research: Some basic principles were observed and R&D has started, but applications are still speculative.
Examples of possible EU funding programmes: EIC Pathfinder of Horizon Europe
Definition of this TRL level:
Software: To reach this TRL level you need to have experimental and analytical studies conducted to validate the predictions on the technology. These studies provide a preliminary technology proof of concept, performed in the laboratory.
Medical research: Experimental and analytical studies were conducted to validate the predictions on the technology. These studies provide a preliminary technology proof of concept, performed in the laboratory. In biomedical technologies, ’in vitro’ tests fit in this stage.
Examples of possible EU funding programmes: EIC Pathfinder of Horizon Europe
Definition of this TRL level:
Software: At this stage, the basic technological components are designed, developed and integrated to verify that they work together. In the case of software products, this is the stage of ‘alpha tests’.
Medical research: At this stage, the basic technological components are designed, developed and integrated to verify that they work together.
Examples of possible EU funding programmes: EIC Pathfinder, EIC Transition of Horizon Europe
Definition of this TRL level:
Software: Integration of the technological components and testing of their applications in a realistic environment. This step corresponds to the validation of system or process components in software products.
Medical research: Integration of the technological components and testing of their applications in a realistic environment. This step corresponds to the pre-clinical trials in the pharma sector.
Examples of possible EU funding programmes: EIC Transition of Horizon Europe
Definition of this TRL level:
Software: Evaluation of the prototype or representative model in a relevant environment. This step corresponds to a ’beta’ version.
Medical research: Evaluation of the prototype or representative model in a relevant environment. In the pharmaceutical sector, this step corresponds to the first phase of clinical trials, while in the medical devices sector, it corresponds to the safety demonstration of the device.
Examples of possible EU funding programmes: EIC Transition, EIC Accelerator, Clusters’ and Partnership’s RIA calls, IA calls, KDT calls, CBE calls of Horizon Europe
Definition of this TRL level:
Software: Evaluation of the prototype in a operating environment, similar to the real one. The final prototype product design and testing.
Medical research: Evaluation of the prototype in an operating environment, similar to the real one. The second phase of clinical trials fits in this TRL as well as the final prototype product design and testing in medical device
Examples of possible EU funding programmes: EIC Accelerator, Clusters’ and Partnership’s IA calls, KDT calls, CBE calls of Horizon Europe
Definition of this TRL level:
Software: In a real system, the technology proved to be in accordance with the conditions specified. In software, it corresponds to a pre-commercial demonstration.
Medical research: In a real system, the technology proved to be in accordance with the conditions specified. In biomedical projects, this stage corresponds to the third phase of clinical trials.
Examples of possible EU funding programmes: EIC Accelerator, Clusters’ and Partnership’s IA calls, KDT calls, CBE calls of Horizon Europe, Innovation Fund
Definition of this TRL level:
Software: The system incorporates the new technology in its final form and had applied it in actual application conditions. It is ready for commercialisation.
Medical research: The system incorporates the new technology in its final form and had applied it in actual application conditions. It is ready for commercialisation.
Examples of possible EU funding programmes: Innovation Fund